Key Takeaways
- Misoprostol and mifepristone, the two drugs used as part of the FDA approved medication abortion regimen, also have a wide range of clinical applications and are used to manage the reproductive health of millions of women across the nation that include but are not limited to abortion.
- Louisiana has enacted and implemented a law reclassifying mifepristone and misoprostol as Schedule IV controlled substances. Similar legislation has been introduced in Texas, Missouri, and Kentucky.
- Among a sample of claims weighted to represent people with employer sponsored insurance, over half of reproductive age women with an outpatient claim for misoprostol unrelated to abortion did not have a pregnancy related diagnosis and 28% were prescribed the medication to manage a miscarriage.
- About half of outpatient claims for misoprostol in Texas and Missouri Medicaid, and a third in Kentucky and Louisiana were used to manage miscarriages. About one in five were used for other pregnancy-related reasons, such as induction of labor or to manage pregnancy-related hemorrhages in Texas, Missouri, and Louisiana Medicaid.
- States seeking to reclassify mifepristone and misoprostol as controlled substances to limit access to medication abortion may impede access to medications that impact the health and safety of women who rely on these medications to manage a wide range of conditions.
On October 1, 2024, Louisiana enacted a new law that reclassifies two drugs commonly used for medication abortion, mifepristone and misoprostol, as Schedule IV controlled substances. Soon after, lawmakers in Texas, Missouri, and Kentucky proposed similar legislation to designate these medications as controlled substances. Typically, only drugs with the potential for dependency or abuse are classified as controlled substances, with their scheduling (I-V) determined by their level of abuse risk. Examples of Schedule IV drugs include Ambien and Xanax. In addition to abortion care, mifepristone and misoprostol are used to treat several medical conditions. As controlled substances in Louisiana, they require special storage, ordering, and documentation procedures, which could result in delays in access to emergency and routine outpatient care. The other impact of these kinds of laws is to cause clinicians and pharmacists to be hesitant to prescribe or dispense these medications for non-abortion related care as to avoid becoming a formal part of the state record tracked through the Louisiana Prescription Monitoring Program (PMP).
Using data from both a convenience sample of claims weighted to be representative of people with employer sponsored health insurance and Medicaid claims from Texas, Missouri, Kentucky, and Louisiana, this analysis finds that these medications are often provided for many reasons other than abortion. Laws that limit access to these drugs could particularly affect women who rely on these medications to manage miscarriages, get IUDs, manage ulcers or autoimmune conditions as well as the clinicians that serve them.
Indications for Use of Misoprostol and Mifepristone
Both misoprostol and mifepristone are classified as essential medicines by the World Health Organization (WHO). Misoprostol is frequently used in obstetrics and gynecology for procedures like the medical management of miscarriage, induction of labor, cervical ripening before surgical procedures, and the treatment of postpartum hemorrhage. It is a prostaglandin that causes cervical softening and dilation, as well as uterine contractions. It is also indicated for reducing the risk of nonsteroidal anti-inflammatory drug (NSAIDs like aspirin and ibuprofen) induced gastric ulcers in patients at high risk of complications. Either alone or in combination with mifepristone, misoprostol can be used to terminate an intrauterine pregnancy in early stages.
Mifepristone, the other drug included in the abortion medication regimen approved by the Food and Drug Administration (FDA), works by blocking the hormone progesterone. And while most patients who receive medications for miscarriage management are given misoprostol alone, the combination of mifepristone and misoprostol has been shown to be more effective in miscarriage management and is the recommended protocol by The American College of Obstetricians and Gynecologists (ACOG). In addition, mifepristone is also indicated for controlling hyperglycemia in adults with Cushing syndrome.
Use of Misoprostol and Mifepristone
The analysis examines the outpatient diagnoses of reproductive aged females, ages 15-49, who had a non-abortion related misoprostol or mifepristone prescription or outpatient claim using the 2023 Merative MarketScan Encounter Database and 2021 Medicaid claims. These medications are often used for inpatient pregnancy-related procedures, such as miscarriage management, labor and delivery, as well as emergency situations such as hemorrhage. However, a limitation of this analysis was that it was not able to identify misoprostol or mifepristone use in an inpatient setting due to the bundling of drug claims along with other inpatient procedures for reimbursement purposes. The findings in this analysis thus include only the provision of the medications in an outpatient setting. Additionally, the claims used in this analysis only include medications for which there were claims that were submitted to the benefit plan and do not include services for which a claim was not filed. As this most directly impacts circumstances when mifepristone and misoprostol were used for medication abortions that were paid for out-of-pocket without using insurance and could result in an underestimate of the share of individuals using these drugs for the purposes of terminating a pregnancy, this analysis focuses only on medications provided via prescription or outpatient claim for non-abortion related care.
Among those with a non-abortion related mifepristone or misoprostol claim, the following outpatient diagnoses were flagged if they were within 90 days of the mifepristone or misoprostol claim because these drugs can be provided a month or two prior to a procedure:
- miscarriage
- other pregnancy-related conditions (including hemorrhage, induction of labor, delivery)
- non-pregnancy related cervical ripening (IUD insertions, polyps, noninflammatory disorders of cervix uteri)
- other non-pregnancy-related conditions for which misoprostol or mifepristone are used (including rheumatoid arthritis, fibroids, gastric ulcers, dermatitis, low back pain, osteoarthritis, and Cushing syndrome)
Because some patients had diagnoses in more than one of these categories, a hierarchy was applied to classify health plan enrollees into one category: miscarriage, other pregnancy-related, non-pregnancy-related cervical ripening, and other non-pregnancy-related conditions. Individuals with a mifepristone or misoprostol claim that did not have a diagnosis from one of these categories within 90 days of their claim or individuals that only had prescription claims for mifepristone or misoprostol without outpatient claims within 90 days were classified as “unexplained.”
Misoprostol Use
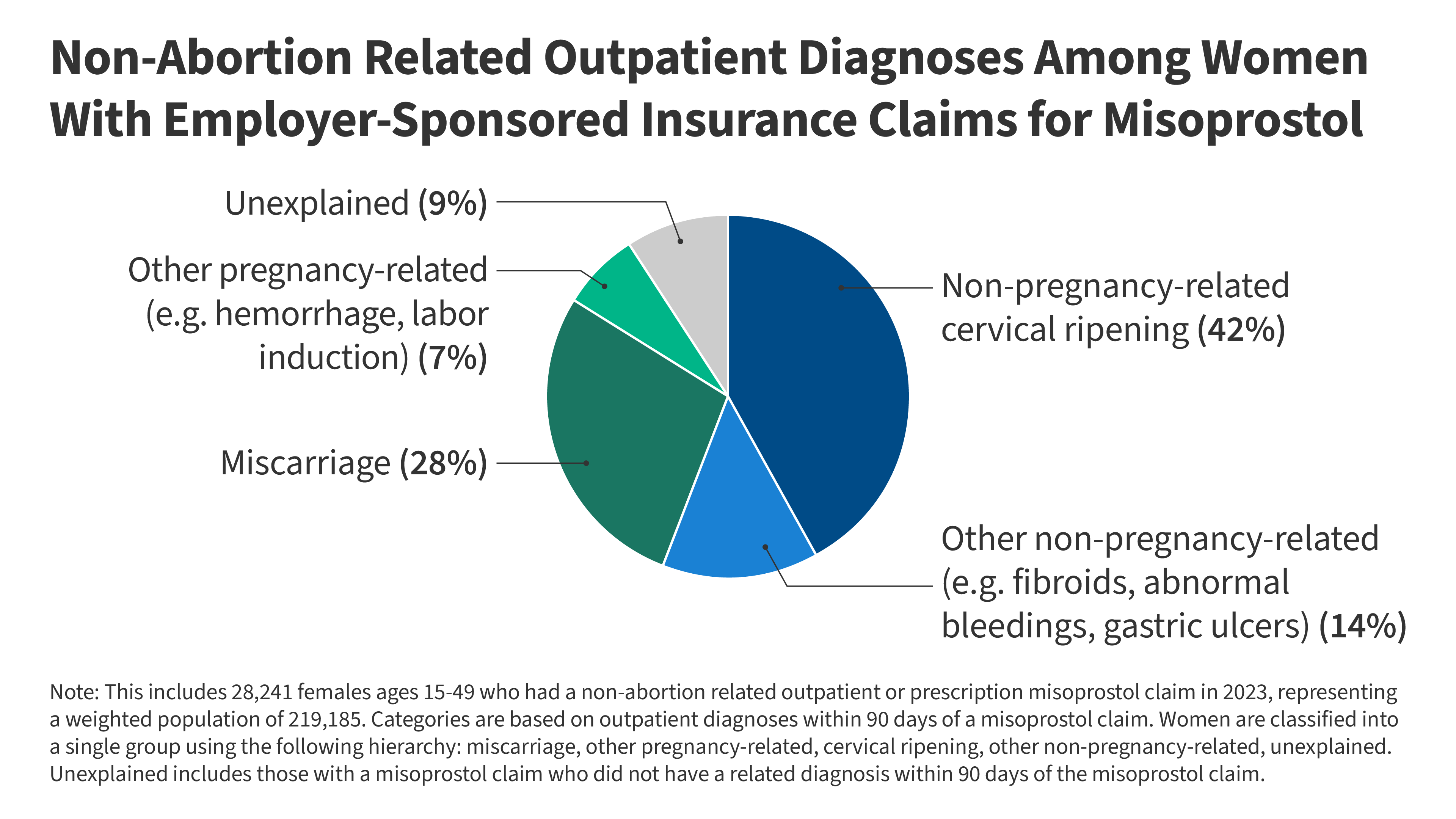
Among the reproductive aged women enrolled in employer-sponsored plans who had a non-abortion related outpatient misoprostol claim, 65% had no indication of pregnancy within 90 days of their misoprostol claim date (Figure 1). Diagnoses for conditions where misoprostol would be indicated for non-pregnancy-related cervical ripening, such as for an IUD insertion, made up the largest share of those with a non-abortion related outpatient or prescription misoprostol claim (42%). Another 14% had a diagnosis for another non-pregnancy related reason for which misoprostol can be used, such as gastric ulcers, rheumatoid arthritis, fibroids, or other conditions related to abnormal bleedings. More than one in four (28%) had a miscarriage diagnosis within 90 days and 7% had an outpatient diagnosis for another pregnancy-related condition, such as maternal hemorrhage or labor induction. The remaining 9% of women with an outpatient misoprostol claim did not have a diagnosis related to one of these conditions within 90 days and were categorized as unexplained.
Similarly, about three in ten Medicaid beneficiaries in Kentucky and Louisiana had a miscarriage diagnosis within 90 days of their misoprostol claim. In contrast, among Texas and Missouri Medicaid beneficiaries, around half of misoprostol claims had a miscarriage diagnosis within 90 days of the claim, likely because misoprostol is commonly used to expedite the expulsion of nonviable pregnancy tissue in miscarriage management (Figure 2). About one in five beneficiaries in Texas, Missouri, and Louisiana had another pregnancy-related diagnosis within 90 days, which could have included hemorrhage or labor induction.
About a third of beneficiaries in Texas and Missouri did not have a pregnancy diagnosis within 90 days of their misoprostol claim, while over half of beneficiaries in Kentucky and Louisiana did not. Around one in ten beneficiaries in Texas, Missouri, and Louisiana had a claim for non-pregnancy-related cervical ripening typically indicated for an IUD insertion or stricture or stenosis of the cervix for which misoprostol may be used to facilitate IUD insertion. However, over a quarter of beneficiaries in Kentucky had a claim for non-pregnancy-related cervical ripening within 90 days of their misoprostol claim. One in five beneficiaries in Kentucky had other non-pregnancy-related diagnoses for which misoprostol is used, such as gastric ulcers, fibroids, and abnormal bleeding, while this share was smaller in Texas, Missouri, and Louisiana, but still around 10%.
Mifepristone Use
In contrast to misoprostol, the vast majority of women with non-abortion related outpatient claims for mifepristone (94%) had a miscarriage diagnosis within 90 days of their mifepristone claim (Figure 3). The remaining 6% had a diagnosis for another condition, such as Cushing syndrome.
Implications for Access
Misoprostol and mifepristone have clinical applications that are used to manage the reproductive health of millions of women across the nation. Despite their use as effective medications for terminating pregnancies, they are also frequently prescribed to manage many other conditions outside of abortion. Laws that seek to restrict access to these drugs will also impact healthcare for reproductive aged women who rely on them to treat a range of non-abortion related conditions, especially in states where abortion is already banned. Because controlled substances require specific prescribing and dispensing protocols and documentation outside of clinical records, health care providers, and pharmacies may choose not to stock or dispense them to avoid the risk of penalties or criminalization. Healthcare providers must also be licensed with the Drug Enforcement Administration (DEA) in order to prescribe controlled substances which could cause a further chilling effect in their use, despite the robust evidence of their effectiveness and safety. This could affect women who are prescribed misoprostol for procedures such as IUD insertion or uterine polyp removal, but it also jeopardizes effective and potentially life-saving miscarriage management for the up to one-in-five pregnancies that are estimated to result in early pregnancy loss.
Beyond outpatient care, these barriers could also result in treatment delays in emergency situations such as hemorrhage during labor and delivery, which accounts for 14% of pregnancy-related deaths in the United States. Under the new Louisiana law, hospitals must now securely lock away mifepristone and misoprostol or use a passcode-protected dispensing system. Some resource-limited hospitals, such as rural or community hospitals, may also lack the capacity to stock controlled substances, making these medications unavailable to patients at those facilities. States like Louisiana, Texas, Kentucky and Missouri that seek to reclassify mifepristone and misoprostol as Schedule IV controlled substances may not only limit access to medication abortion, but they may also have consequences that impact the health and safety of many who need these medications for reasons other than terminating a pregnancy.
Methods
The analysis is based on prescription drug and outpatient medical claims from the 2023 Merative MarketScan Commercial Claims and Encounters Database, which contains claims information provided by employer-sponsored plans, and 2021 Transformed Medicaid Statistical Information System Analytic File (TAF) data from Texas, Missouri, Kentucky, and Louisiana. There were 6,106,254 total women of reproductive age in the 2023 Merative MarketScan Commercial Claims and Encounter Database representing a weighted population of 47,235,846 women of reproductive age. The 2021 Transformed Medicaid Statistical Information System Analytic File included women of reproductive age from Texas (n = 1,625,479), Missouri (n = 337,305), Kentucky (n = 501,500), and Louisiana (n = 551,103).
Reproductive aged women, ages 15-49, were included if they had a non-abortion related misoprostol or mifepristone prescription or outpatient claim. National Drug Codes (NDC) were used to identify misoprostol and mifepristone in prescription claims, while Healthcare Common Procedure Coding System (HCPCS) codes S0190 and S0191 were used to identify outpatient provision. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes and Current Procedural Terminology (CPT)/HCPCS procedure codes were used to classify conditions from outpatient claims within 90 days of a misoprostol or mifepristone claim date. We chose 90 days because misoprostol can often be prescribed a month or two before a procedure, especially prior to LARC insertions. Women with a misoprostol or mifepristone claim who had multiple diagnoses within this period were categorized according to a mutually exclusive hierarchy as follows: miscarriage, other pregnancy-related conditions, cervical ripening, other non-pregnancy-related conditions. If no diagnosis from one of these categories was found within 90 days of the drug claim, they were included in the unexplained category. A small group of women (1%) with a misoprostol prescription claim who did not have an outpatient claim within 90 days were also included in the unexplained category. To make MarketScan data representative of enrollment in employer-sponsored insurance health plans nationally, weights were applied to match counts in the Current Population Survey for enrollees and their dependents employed by firms of a thousand or more workers by sex, age and state.
Appendix
Publisher: Source link